TE-821...
」
告別巨痛針!免疫功坊TE-8214:從「麥芽糖」到「水溶液」的長效藥物革命
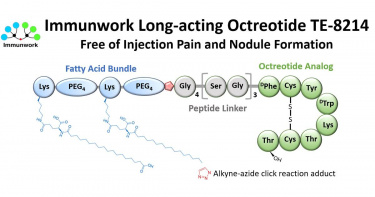
長效型胜肽藥物的發展,是現代醫學的一大福音,它顯著改善了病患的生活品質。然而,對於「生長激素分泌異常的相關徵兆」的病患來說,儘管現有治療藥物奧曲肽(Octreotide)已能緩解病徵,但長效型製劑的注射疼痛卻是難以言喻的夢魘。在【生活講堂】節目中,主持人—高葳(Stacey) 專訪了台灣免疫功坊(Immunwork)董事長、被譽為「台灣抗體之父」的張子文博士。張博士在訪談中深入揭示,他們正在研發的創新藥物TE-8214,目標就是要徹底解決這個困擾病患數十年的痛點。舊藥之痛:麥芽糖般的黏稠與結節為了將奧曲肽從原本只有1.5小時的短暫半衰期,延長到能數週注射一次的長效療程,現行市場上的長效緩釋劑(如 Sandostatin LAR)必須採用 Depot(儲藏物)的概念。張博士在節目中生動地形容,這種緩釋劑的劑型,在調製出來後,會變成一種「很黏稠的東西,看起來有點像麥芽糖」。這種極端的黏稠度帶來了災難性的後果:1. 粗針深層注射: 由於藥物黏稠難推,病患必須使用極粗的針頭(例如 18G 或 19G),並深層推入臀部肌肉中。張博士提及,有醫師反應,光是看到這種粗針,有的病患就可能害怕得想跑掉。2. 痛苦結節: 藥物在肌肉內釋放時,會在注射部位形成難以消散的硬塊或結節。這類硬塊可能需要數個月甚至一年才能緩慢消退,嚴重影響病患的日常活動和生活品質。新藥突破:脂肪酸束與白蛋白攜帶的長效機制免疫功坊的TE-8214代表了新一代長效藥物在技術與人文關懷上的雙重突破。張博士指出,TE-8214 運用公司獨有的「脂肪酸束的技術」對奧曲肽進行了全新的修飾,創造出獨步全球的創新藥物。最大的突破,在於劑型的徹底改變:TE-8214不再依賴聚合物形成的網狀結構來緩釋藥物,它本身就是水溶液型態。由於是水溶液,TE-8214可以使用極細的針頭(例如 30G,如同疫苗針般細)進行皮下注射,不再需要痛苦的肌肉深層注射,徹底減輕病患的疼痛。長效機制也更為精妙:TE-8214在被皮下注射後會迅速進入血液系統,並與血液中的白蛋白(Albumin)結合。白蛋白作為體內的攜帶者,將藥物攜帶在血液中,使半衰期延長。這種機制既達到了長效的目的,同時也完全避免了肌肉結節的產生。在專訪的最後,張子文博士強調,他的新藥研發不僅追求有效性,更追求人性化。TE-8214目前正在人體臨床試驗階段,一旦成功上市,將為病患提供一個「無痛、細針、不結節」的治療新選擇,讓病患能夠更積極、更有尊嚴地配合治療。
T-E Pharma旗下免疫功坊長效型奧曲肽新藥TE-8214 第一期臨床試驗證實有效改善注射不適、抑制發病生化指標IGF-1
T-E Pharma旗下之免疫功坊 (Immunwork, Inc.) 今日宣布,其自主研發的長效型奧曲肽新藥TE-8214已於澳洲順利完成第一期臨床試驗。結果顯示TE-8214具備良好的安全性與耐受性、可達成預期藥理機制,且皮下注射輕易,不產生施打部位副作用。TE-8214為免疫功坊運用專利平台技術開發之長效型奧曲肽 (octreotide) 類似物,經由在奧曲肽分子上連結含有兩條脂肪酸鏈的脂肪酸束,達成延長藥物半衰期,可應用於治療肢端肥大症與多種神經內分泌腫瘤所引致的病徵。上述兩類疾病常伴隨嚴重的內分泌失調,導致消化性潰瘍、嚴重腹瀉、氣喘、組織異常生長、關節疼痛與高血壓等症狀,若未妥善治療,將大幅影響患者的生活品質與壽命。(圖/免疫功坊提供。)隨著全球對奧曲肽藥物需求的增長,近年來相關市場規模持續擴大,預估2030年將達100億美元。現有市售與研發中的相關產品,多為高黏稠度微粒或凝膠狀緩釋劑型,須使用19G或20G的粗針頭進行肌肉 (如Octreotide LAR) 或深層皮下注射 (如Lanreotide Autogel),不僅施打困難,更易引發注射部位疼痛及結節等副作用,造成病患對治療的接受度與依從性明顯下降。(圖/免疫功坊提供。)免疫功坊創辦人兼執行長,先前發明多項創新藥物的免疫學家張子文表示:「TE-8214 完成第一期臨床試驗是免疫功坊的重要里程碑,也為肢端肥大症與神經內分泌腫瘤患者帶來新希望。TE-8214具備高水溶性,可透過30G細針頭進行皮下注射,大幅減輕患者注射時的疼痛與恐懼感。未來我們將憑藉此關鍵優勢,積極拓展全球奧曲肽市場,為患者提供更安全、更舒適的治療選擇。」TE-8214的第一期臨床試驗採雙盲、單劑量遞增設計,設有四個劑量組別 (0.6 mg、1.2 mg、2 mg及4 mg),每組納入8名健康受試者,其中6人接受TE-8214,2人接受安慰劑(生理食鹽水)。整體結果顯示TE-8214耐受性良好,僅出現輕微不良反應,未觀察到任何嚴重不良事件。常見的奧曲肽副作用為消化道不良反應,本試驗24位TE-8214受試者中,僅3人出現極輕微的消化道不適,證實TE-8214的安全性與耐受性表現優異。更重要的是,所有TE-8214受試者均未出現注射部位疼痛或結節情形,結果與安慰劑組的受試者完全相同,顯示TE-8214的劑型設計成功解決緩釋劑型的痛點問題。此外,奧曲肽類藥物主要透過抑制體內類胰島素生長因子 (Insulin-like growth factor 1, IGF-1) 濃度發揮治療效果。在本次試驗的高劑量組別 (2 mg和4 mg) 中,12位TE-8214受試者中有10位 (83%),其IGF-1濃度下降超過20%,顯示TE-8214具備顯著的IGF-1抑制效果,進一步支持其作為治療用藥之優秀潛力。目前,免疫功坊正積極展開第二期臨床試驗的籌備工作,預計於2025年第三季在台灣正式啟動。我們期盼透過未來階段的試驗推進,加速驗證TE-8214的治療潛力,拓展其臨床應用與全球市場佈局,為更多患者帶來突破性的治療方案。TE-8214與市售長效型奧曲肽類藥物的差異化比較(圖/免疫功坊提供。)關於免疫功坊免疫功坊位於國家生技研究園區,專注於開發結合標的 (T) 與效應(E) 結構單元的「T-E型藥物」,以實現兼具療效與安全性的突破性治療。公司運用自主研發的「脂肪酸束平台」技術,目前已有兩項新藥進入臨床階段,並有多項臨床前候選藥物,未來將持續推進創新藥物研發與臨床應用。聯絡資訊若您有合作洽談需求或欲了解更多資訊,歡迎與我們聯繫。電子郵件:bd@immunwork.com公司官網:https://www.immunwork.com/公司影片:https://www.youtube.com/watch?v=1owxqn08HoQT-E Pharma's subsidiary Immunwork Announces Positive Phase I Top-Line Data for TE-8214, a Next-Generation Long-Acting Octreotide, Demonstrating Favorable Safety, Tolerability, and Potent IGF-1 Suppression.·Pioneering Patient Experience: TE-8214, a highly water-soluble aqueous solution, enables pain-free subcutaneous injection with a thin 30G needle, eliminating injection site reactions seen with current therapies.·Strong Pharmacodynamic Activity: Robust, dose-dependent reduction of disease biomarker IGF-1, with 83% of high-dose participants achieving a >20% reduction, confirming therapeutic potential.·Significant Market Potential: Positioned for the US$10 billion long-acting octreotide market by addressing key unmet needs of painful injections and side effects associated with current viscous, thick-needle formulations.·Phase II Initiation and Strategic Partnering: Phase II trial planned for Q3 2025; Immunwork is actively seeking strategic collaborations to accelerate global development and commercialization.Immunwork, Inc., a clinical-stage biotechnology company developing transformative therapies, today announced positive top-line results from its Phase I clinical trial (in Australia) of TE-8214, a novel long-acting octreotide analog for the treatment of acromegaly and neuroendocrine tumors (NETs). The study met its primary objectives: TE-8214 demonstrated good safety, tolerability, and pharmacological activity, with pain-free subcutaneous administration and no injection site reactions.(This photo provided by Immunwork, Inc.)A Next-Generation Therapy for Unmet Patient NeedsTE-8214 was developed using Immunwork's proprietary fatty acid modification platform, which extends the drug's half-life and increases water solubility. TE-8214's water-soluble, low-viscosity formulation allows for subcutaneous administration with a fine 30G needle, offering a dramatically improved patient experience compared to the current standard-of-care, which requires thick, viscous formulations administered with large 19G or 20G needles that often cause significant pain and injection site reactions. This innovation addresses a critical need for safer, more patient-friendly long-acting therapies in a global octreotide market projected to reach US$10 billion by 2030.(This photo provided by Immunwork, Inc.)Phase I Clinical Highlights TE-8214's Differentiated ProfileThe randomized, double-blind, placebo-controlled, single-ascending dose study enrolled 32 healthy subjects (24 receiving TE-8214 and 8 receiving saline) across four dose cohorts (0.6, 1.2, 2, and 4 mg). Exceptional Safety and Tolerability: TE-8214 was well tolerated with no serious adverse events reported. Notably, no TE-8214 recipients experienced injection site pain or nodules—results identical to placebo with saline — demonstrating a clear advantage over existing therapies. Gastrointestinal side effects, often seen with octreotide, were minimal: only 3 of 24 TE-8214 recipients reported very mild gastrointestinal discomfort. Potent and Sustained Pharmacodynamic Activity: Octreotide drugs mainly exert their therapeutic effects by suppressing insulin-like growth factor 1 concentration in the body. In the two highest dose cohorts (2 mg and 4 mg), 10 of 12 participants (83%) achieved a >20% reduction in insulin-like growth factor 1, confirming robust biological activity and validating its therapeutic potential for Phase II studies.“These Phase I clinical trial results confirm TE-8214's differentiated profile: TE-8214 is highly water-soluble, can be administered subcutaneously using a fine needle, significantly reducing injection pain and side effects for patients,” said Dr. Tse-Wen Chang, founder and CEO of Immunwork and a pioneer of anti-CD3 (OKT3) and the inventor of anti-IgE (Xolair) for asthma and allergy. “By solving the fundamental formulation and administration challenges of current treatments, we have created a product with the potential to become the new standard of care. TE-8214's profile—combining ease of administration, superior tolerability, and strong efficacy—positions it to capture a significant share of the market and, most importantly, vastly improve the quality of life for patients.”Strategic Outlook: Advancing to Phase II and Seeking Partnerships Immunwork is preparing for a Phase II trial of TE-8214 in Taiwan, planned to start in Q3 2025, to further evaluate the efficacy and safety of TE-8214 in patients. The company is actively pursuing strategic partnerships with pharmaceutical leaders to accelerate late-stage clinical development, navigate global regulatory pathways, and maximize the commercial potential of TE-8214.TE-8214: A Differentiated Profile vs. Marketed Long-acting Octreotide Analogs.(This photo provided by Immunwork, Inc.)About ImmunworkImmunwork, based in the National Biotechnology Research Park in Taipei, develops "T-E type drugs" that combine targeting (T) and efficacy (E) moieties for transformative therapies with enhanced efficacy and safety. Leveraging proprietary fatty acid bundle technologies, Immunwork has two clinical-stage candidates and a robust preclinical pipeline, and is committed to advancing innovative medicines for global unmet needs.Contact InformationFor partnership inquiries and further information:E-mail:bd@immunwork.comWebsite:https://www.immunwork.com/Video: https://www.youtube.com/watch?v=1owxqn08HoQ